Making Research Possible
The CRU provides expert nursing, study coordination and dedicated facilities for patient-oriented research, including outpatient, inpatient and ambulatory studies.
Most of our registered nurses (RNs) have graduate or terminal degrees. All of our full-time RNs:
-
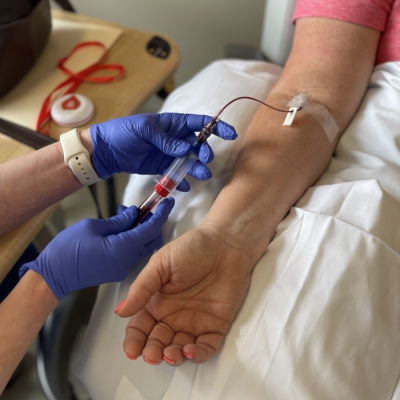
are highly proficient at intravenous starts and phlebotomy.
-
are highly proficient at assisting with bone, fat, and muscle biopsies and specialized CLAMP studies.
-
are telemetry and radiation safety trained.
-
have ACLS certification.
-
have PALS certification.
We Provide:
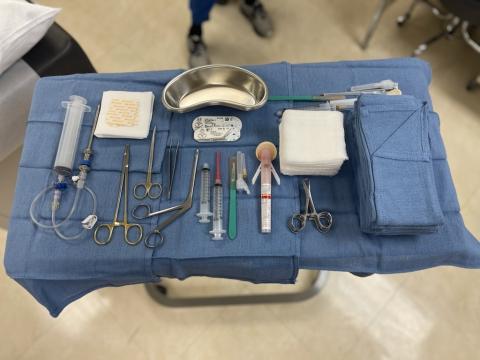
Inpatient and outpatient units with expert clinical research staff, lab processing and shipping

Including expert clinical staff, experienced coordinators, and community research coordinator/clinical liaison support
The CCTS Outpatient and Inpatient Units are moving to the Ben F. Roach Cancer Care Facility, Floor 3, to enable expanded capacity.
- Phase 1 of the move included the inpatient unit (formerly on on 5 North) and FAABC equipment including the Dexa scanner. These units moved into the new space on November 11, 2024.
- Phase 2 involves the outpatient unit and administrative offices and is scheduled for late summer 2025.
- "Great job team. Really enjoyed my time!"
- Very friendly & competent group. Much appreciated!"
- "The staff always seems very concerned about my comfort as well as the procedure. I am happy to be here."
- "All staff was beyone (sic) exceptional, I will most definitely be interested in more studies. They were all polite, professional and even nice to just talk to. Thank you."
- "Our comfort is paramount, smaller needles were obtained to help w. infusions because of scar tissue buildup, supplies were in room & ready for us when we arrive. Concerns are addressed & changes made if needed. We are made to feel important & necessary to the study & medicine & part of the team. People SMILE and laugh. It's restful to sit back.”
Clinical Research Unit Impact
1140
Average Annual Outpatient Visits
617
Average Annual Inpatient Overnight Visits
55
Current Studies Supported by CRU Nurses and Research Coordinators
Questions about the CCTS Clinical Research Unit? Contact:
Denece Forenback, MSN, BSN, RN, CSN
- Senior Director of Clinical and Nursing Operations
- Denece.Forenback@uky.edu
- 859-323-6481
